This tab contains any automated alerts registered for specific participants of the study. These alerts will have different codes depending on the study in question. As an example, the AESI’s (Adverse Event of Special Interest within a study) being reported below refer to Adverse Events experienced by participants within the study in question, and must be addressed by a clinician.
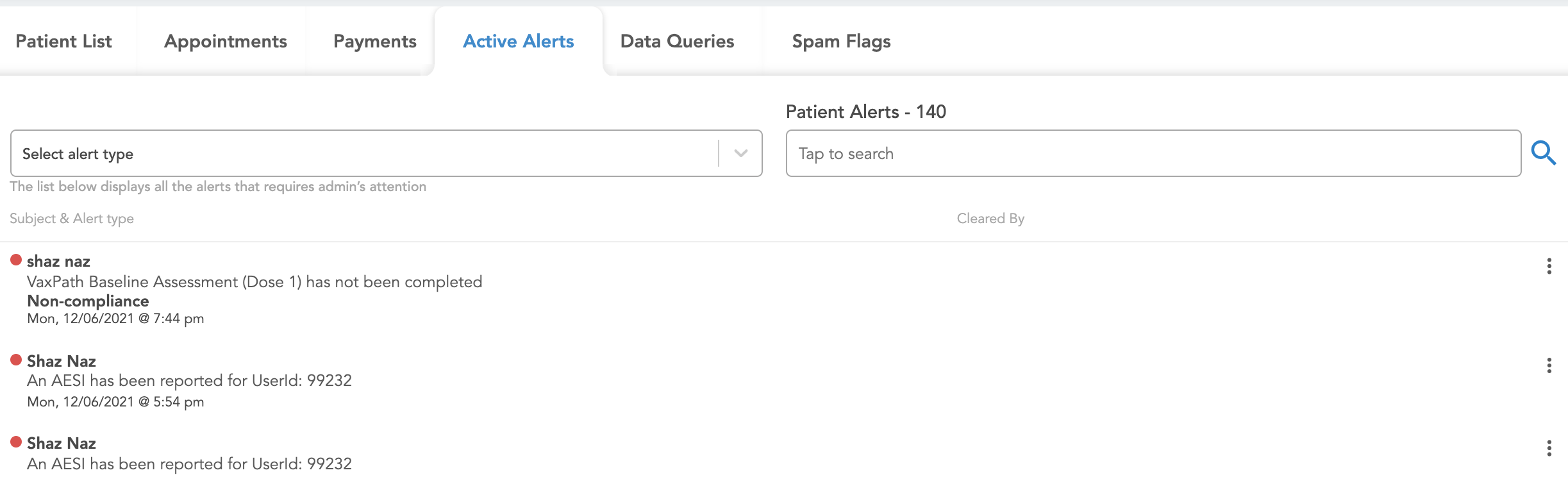
Each alert can be cleared by clicking the three dots on the far right. Afterwards, the name of the admin that cleared it will be visible next to it in the second column.